We manufacture high-precision products and system assemblies with stringent cleanliness requirements. Production takes place in our classified cleanroom environments worldwide for the medical, diagnostics and laboratory sectors, as well as some industrial processes.
Contact us
Sending Email...
SERVICE
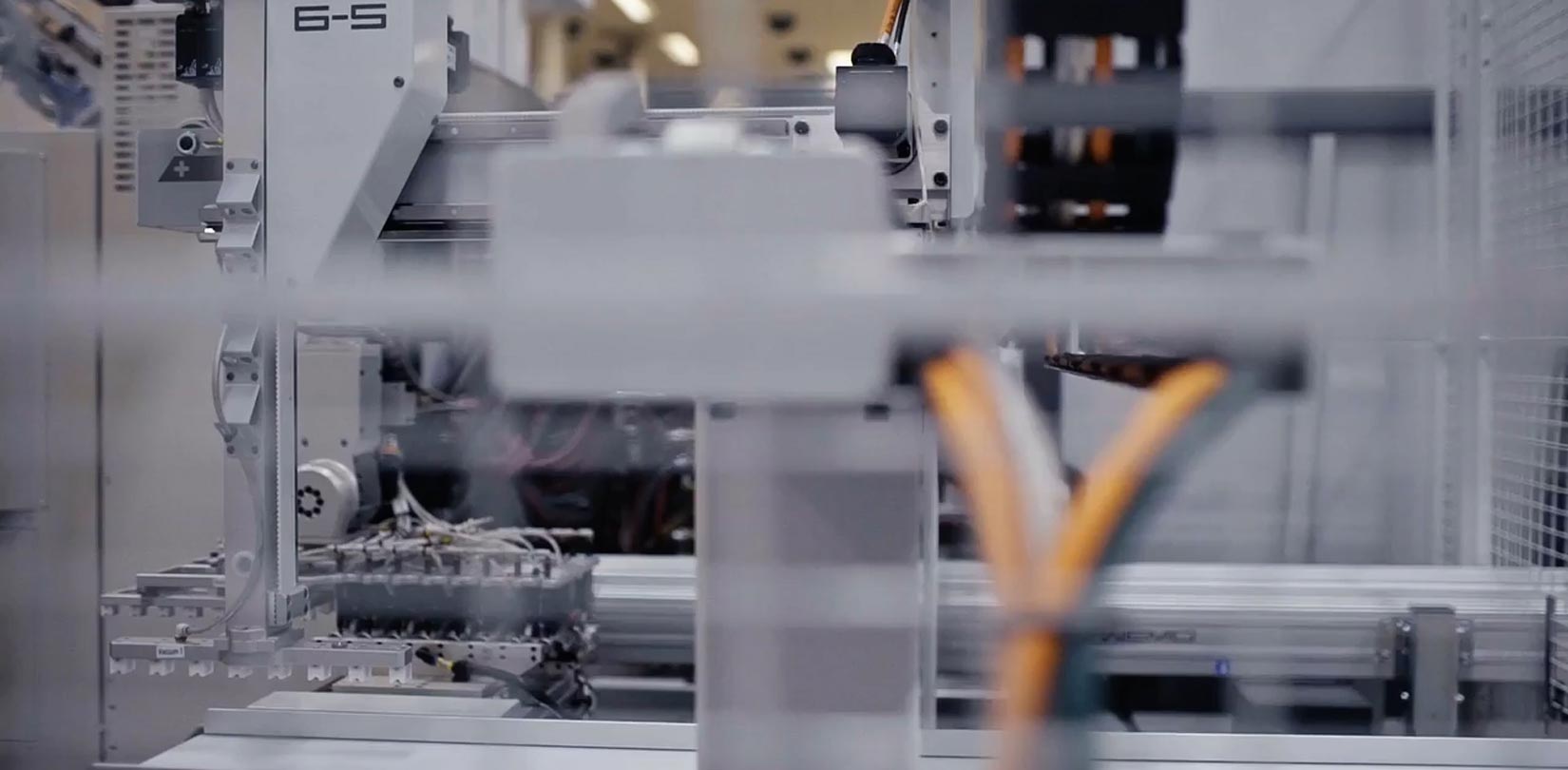
Cleanroom production
Cleanrooms depend on precisely controlled environmental conditions to minimize contamination and protect people and equipment, so their technological infrastructure adheres to the most stringent quality standards.
Controlled classified environment
All Medical Solutions' cleanrooms are classified in accordance with ISO 14644 Class 7 or 8, which means the concentration of viable and non-viable airborne particles, temperature and humidity are controlled. All staff and materials enter through dedicated airlocks, staff are specifically trained to work in these environments and follow special gowning and hygiene procedures when entering and leaving.
Production in controlled non-classified environments
In addition to classified cleanroom production, Nolato offers production in environments that are controlled but not classified. Such manufacturing environments have more basic ventilation and fewer restrictions but still apply best practice to reduce contamination.
Integrated Solutions
Our Integrated Solutions business area uses cleanrooms for processing surface-sensitive components, for example during painting or metallization.
Medical Solutions
Most of Medical Solutions' manufacturing sites offer production in classified cleanroom environments, as this is a prerequisite for products in contact with human tissue or fluids and products used for diagnostic or laboratory purposes.
Microbiological clean room
We offer microbiological and environmentally controlled, contamination-free production without RNase, DNase, human DNA or PCR inhibitors. Products with such requirements are often found in the diagnostics and laboratory sectors.
Maintaining high standards
The infrastructure, machinery and auxiliary equipment inside the cleanrooms adhere to specific cleanroom standards to ensure surfaces can be easily kept clean. Maintenance of cleanroom infrastructure, such as replacement of all HEPA filters, is planned well in advance to minimize the impact on production.

You might also be interested in
MEDICAL EXCELLENCE
Creating world-class operations
Medical Excellence is our way of creating world class operations inspired by the Nolato spirit, our strategies, quality standards and our lean processes and tools. Together, these elements create a framework for managing resources efficiently and increasing value for our customers.
SERVICE
Die-cutting technologies
We offer world leading quality and capacity of traditional and advanced die cut manufacturing in both standard and cleanroom environment.
SERVICE
Decoration technologies
Nolato offers a wide range of technologies to achieve the look you want for your product.