"Not only have Nolato successfully resolved the technical challenges for the customer, we’ve also saved Dräger money by adopting an innovative and smart approach."
Contact us
Sending Email...

CLIENT CASE - MEDICAL SOLUTIONS - DRÄGER
Solving technical challenges
As experts in design, materials and high-volume production, a key aspect of Nolato’s work for its customers is being part of the process of continually improving products. This might involve making changes that enhance the customer experience, make production more efficient or cut manufacturing costs. Or perhaps a combination of all three.
Nolato MediTor has been working for some time with German group Dräger on developing and manufacturing various kinds of tubing and valves for their intensive-care products and breathing apparatus, including respirators and ventilators. The partnership has worked well, which led to Dräger contacting Nolato MediTor to draw on its expertise in materials and manufacturing when they wanted to make improvements to their existing valve unit for ventilating patients.
The valve unit is on the exhalation side of the machines and its purpose is to monitor the patient’s breathing and to close the exhalation channel when the patient breathes in. If the patient is breathing normally, the machine effectively does nothing, but if breathing deteriorates or stops completely, the machine senses this and assists breathing by ventilating the patient to the extent required.
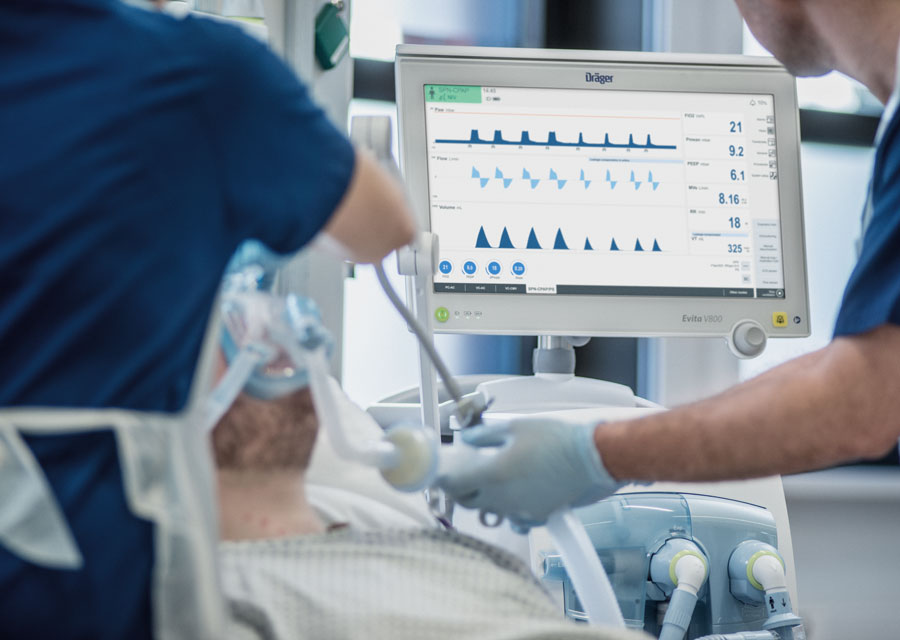
The valve unit is on the exhalation side of Dräger’s respirators and ventilators. Its principal purpose is to sense how the patient is breathing.
Plastic, TPE and silicone
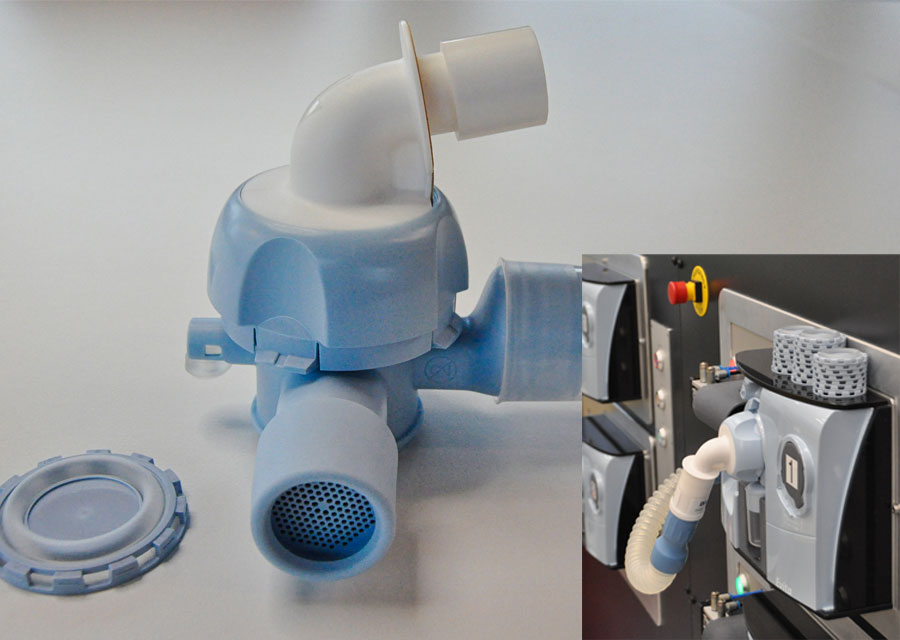
The valve unit comprises nine components made by Nolato MediTor in thermoplastic, TPE and silicone for subsequent assembly on a semi-automated assembly line. The key component of the self-adjusting function is a membrane inside the valve unit that senses breathing pressure.
“In previous versions of the valve unit, this membrane consisted of a metal disc that was overmolded with silicone,” explains Urban Borgcrantz, project manager at Nolato MediTor. “But it wasn’t an optimal solution, as it was quite complicated to manufacture.”
In the new valve unit, the metal membrane has been replaced by a unique injection-molded membrane made of plastic and silicone. The plastic component is formed from a hard polymer that can withstand high temperatures and has excellent dimensional stability, giving it the flatness that Dräger requires.

Nolato MediTor injection-molds nine components in plastic, TPE and silicone. These are assembled on a semi-automated line and undergo thorough inspection before being packaged and supplied direct to Dräger’s customers in the healthcare sector.
Complex 2K injection molding
“We make the membrane using two-component injection molding with rotating molds,” explains Rickard Bengtsson, Technical and Development Engineer at Nolato MediTor. “It’s complex because the membrane consists of three parts: the outer ring and disc in the middle are plastic, while the space between these is made of silicone. If it’s not completely sealed the silicone leaks out and forms flash on the product.
“There was a lot of trial and error, but we had plenty of help from Nolato Medical’s Technical Design Center, TDC, in Hörby for analyses and simulations.”
In-line surface treatment in the mold
The plastic is surface-treated inside the mold to help it bond with the silicone. This is done using a robot, which goes into the mold before it closes and the silicone is molded over. “It’s not unheard of to surface-treat plastic prior to further processing,” says Rickard Bengtsson. “But what’s unusual here is doing it using a robot that goes into the mold and treats the plastic in-line.” Other parts of the valve unit have also been simplified and improved. For example, a fine mesh in one of the valve’s tubes used to require two-component injection molding, but the new valve has just one TPE component that is injection-molded in a single stage.
Once the nine components have been assembled, each valve unit is inspected thoroughly to ensure it functions according to all of Dräger’s requirements.
“We also fit the valve unit with an antenna and RFID label, which contains digitally readable information confirming that it has passed the comprehensive inspections.”
They are then individually packaged and sent direct to end users at hospitals.
Overcoming challenges
“We’re delighted with this project,” says Johan Barkentin, Sales and Project Manager at Nolato MediTor. “Not only have we successfully resolved the technical challenges for the customer, we’ve also saved Dräger money by adopting an innovative and smart approach.”- Nolato MediTor
- Medical Devices
- Medical Excellence
- Medical Solutions
- Injection molding of plastics
- Injection molding of silicone
- Nolato Magasin
- Virtual prototyping
- Technical Design Center
You might also be Interested in
MEDICAL EXCELLENCE
Creating world-class operations
SERVICE
Injection molding of silicone
GROUP COMPANY